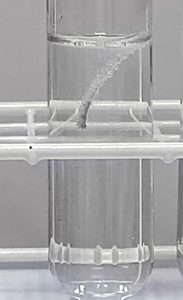
 The aim of this experiment is to find out how changing the concentration of sulphuric acid changes the speed that it reacts with magnesium.
The aim of this experiment is to find out how changing the concentration of sulphuric acid changes the speed that it reacts with magnesium.
When a piece of magnesium reacts with sulphuric acid bubbles of gas are formed. The piece of magnesium gets smaller and smaller until it ‘disappears’. If we time how long it takes to ‘disappear’ we can get some idea of the reaction speed. The longer the time the slower the reaction speed.
Effect of Concentration on Reaction Speed – pupil
Effect of Concentration on Reaction Speed – Teacher/Technician



