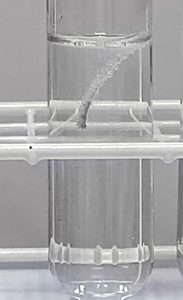
 Different metals react at different speeds with acid. A metal which reacts quickly with acid is called a reactive metal. An unreactive metal reacts only slowly or does not react at all. By finding out how quickly different metals react with acid we can put them in order of reactivity.
Different metals react at different speeds with acid. A metal which reacts quickly with acid is called a reactive metal. An unreactive metal reacts only slowly or does not react at all. By finding out how quickly different metals react with acid we can put them in order of reactivity.
When a metal does react with an acid, bubbles of gas are produced. The speed at which the bubbles are given off tells us how reactive the metal is.
The aim of this experiment is to place the metals, zinc, magnesium and copper in order of reactivity by watching how quickly they react with hydrochloric acid.
Reaction of Metals with Acid – Pupil



