 Anodising is a process used to increase the thickness of the natural oxide layer on the surface of metal parts. In concept it is similar to electroplating in that it involves passing an electric current through a solution which results in a layer being deposited on one of the electrodes.
Anodising is a process used to increase the thickness of the natural oxide layer on the surface of metal parts. In concept it is similar to electroplating in that it involves passing an electric current through a solution which results in a layer being deposited on one of the electrodes.
It is called anodizing because the part to be treated forms the anode electrode of an electrical circuit.
You can find out more about anodising here.
Anodizing increases resistance to corrosion and wear, and provides better adhesion for paint primers and glues than does bare metal.
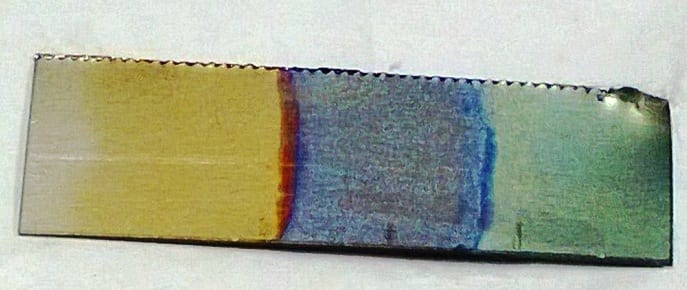
It can also be used for various cosmetic effects, producing a coloured appearence on the metal that is more resistant to corrosion than any paint would be, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add interference effects to reflected light.
In school, there are two viable options for anodising, aluminium and titanium. Anodising aluminium allows dyes to be absorbed into the oxide layer to colour the metal while anodising titanium is an example of structural colour where it is due to interverence caused by the thickness of the layer.



