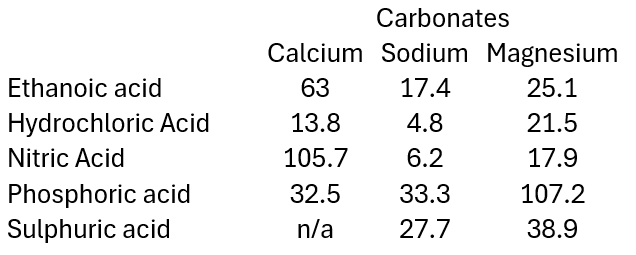
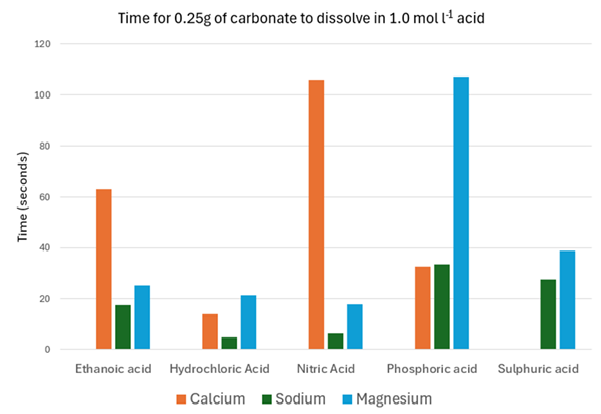
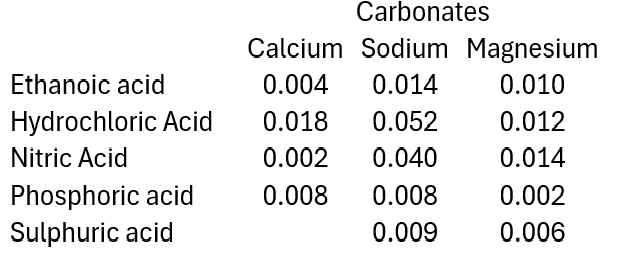
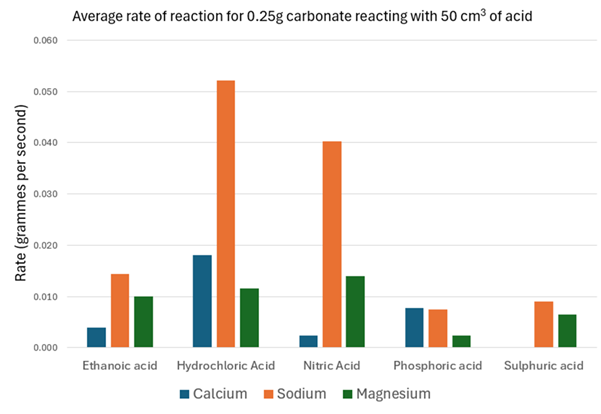
Below you can find some data from various experiments carried out at SSERC involving reacting different combinations of acids and carbonates.
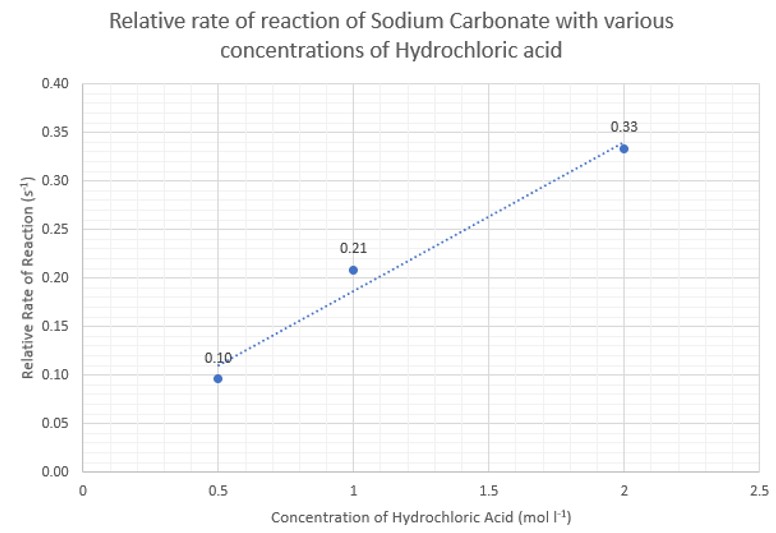
This first section involves the reaction of different carbonates with hydrochloric acid – first simply seeing the time taken for a sample of carbonate to dissolve and then looking at the relative rate of reaction.
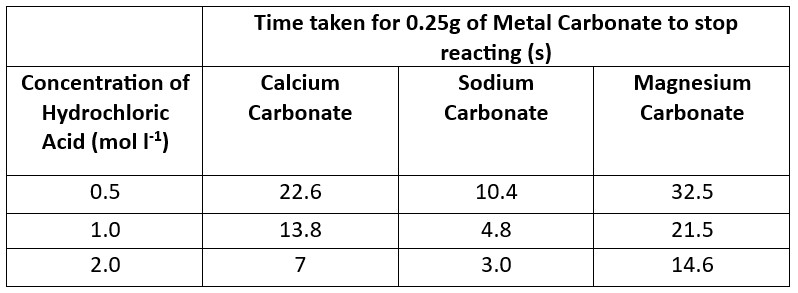
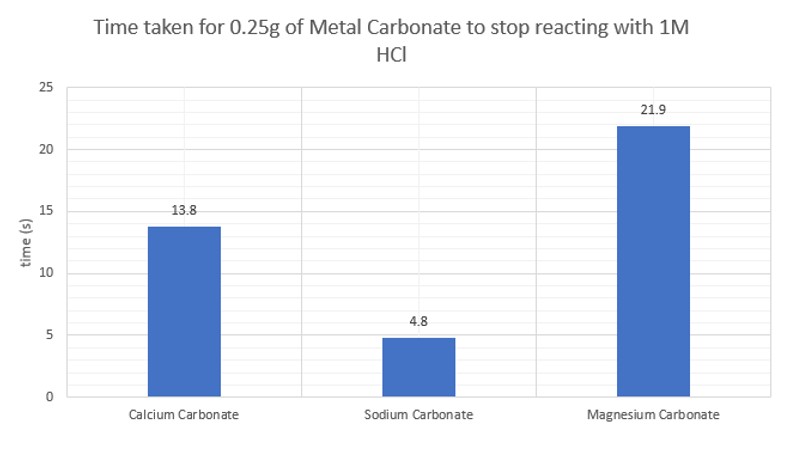
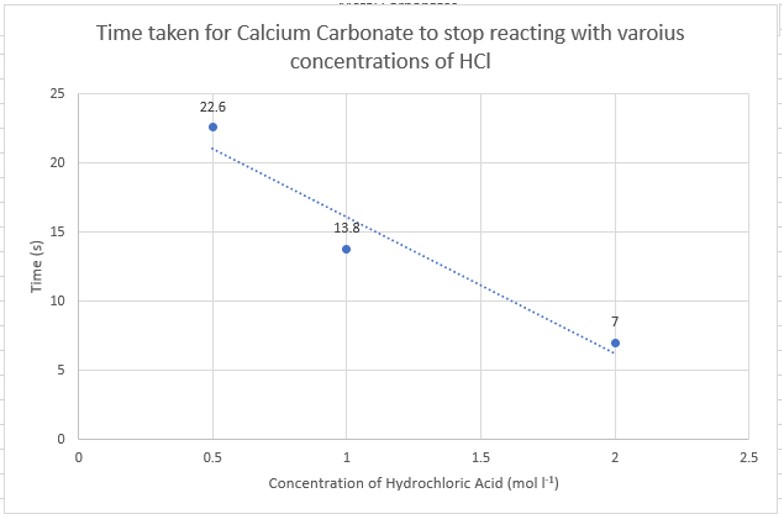
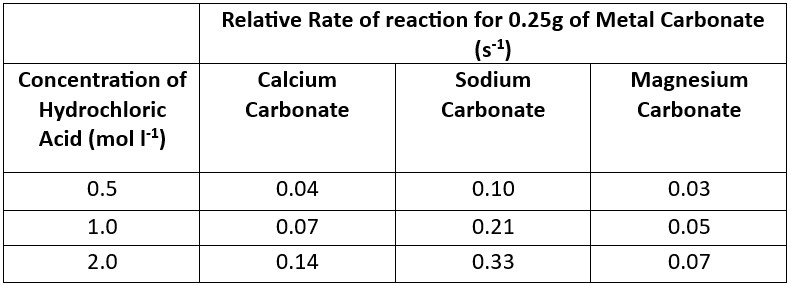
This section looks at the effect of using different acids at the same concentration and temperature: hydrochloric, nitric, sulphuric, phosphoric and ethanoic. All at 1.0 mol l-1)