Materials
- Lab coat
- Eye protection
- Disposable plastic gloves
- Benchkote if necessary
- Disinfectant and paper towels
- Discard jar with disinfectant
- Bunsen burner and mat
- Lens tissues
- Glass slide and coverslip
- Forceps & mounted needle
- Covered beaker containing ethanol
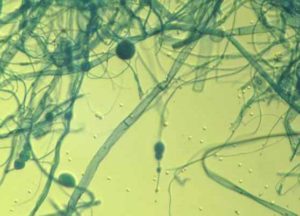
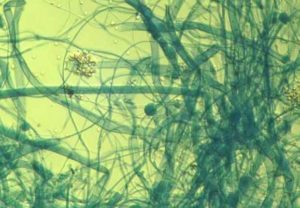
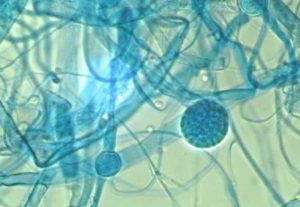
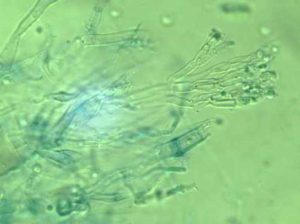
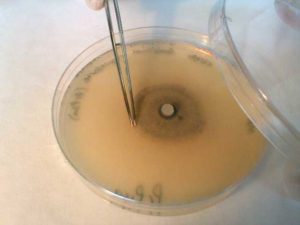
- Plate culture of mould fungus
- Lactophenol blue (note: keep lactophenol off the skin)
- Microscope
 Instructions
Instructions
1. Wear a lab coat, disposable gloves and use eye protection.
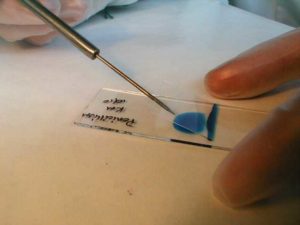
2. Clean slide.
3. Place a drop of lactophenol blue in middle of slide.
4. Flame forceps with ethanol and replace lid.
5. Using aseptic technique, partially lift the lid of the Petri dish and use forceps to remove a small piece of the fungal colony.