Materials
- Lab coat
- Eye protection
- Disposable gloves
- Benchkote if necessary
- Disinfectant and paper towels
- Discard jar with disinfectant
- Fixed smears of bacteria
- Staining rack
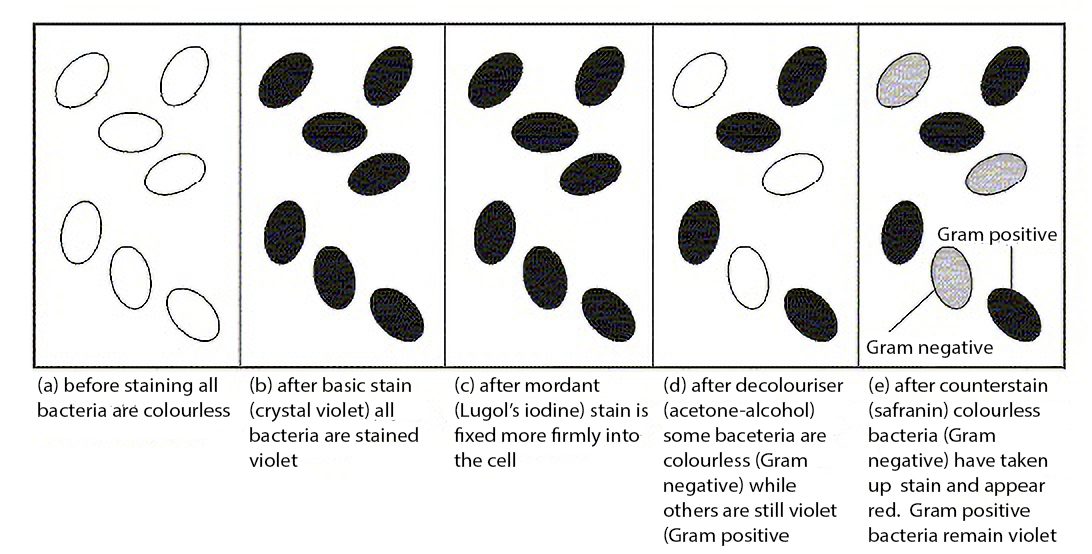
- Crystal violet
- Gram’s iodine
- 95% ethanol
- Safranin
- Labels
- Forceps
- Fibre free blotting paper
- Distilled water bottle
- Bunsen burner and mat
 8. Wash well with water.
8. Wash well with water.
10. Examine under oil immersion if possible. Otherwise, under x600 (x40 objective, x15 eyepiece).
11. Record the Gram reaction (positive or negative), shape (rod, spherical or spiral) and arrangement (clusters, chains or pairs) of the bacteria examined.
12. When finished, dispose of slides into a discard jar.