This article has come about as a result of a meeting at CLEAPSS with a chemistry teacher from Norfolk, David Paterson. His take on it can be found his website[1] and in an article he wrote for Education in Chemistry[2] where he said
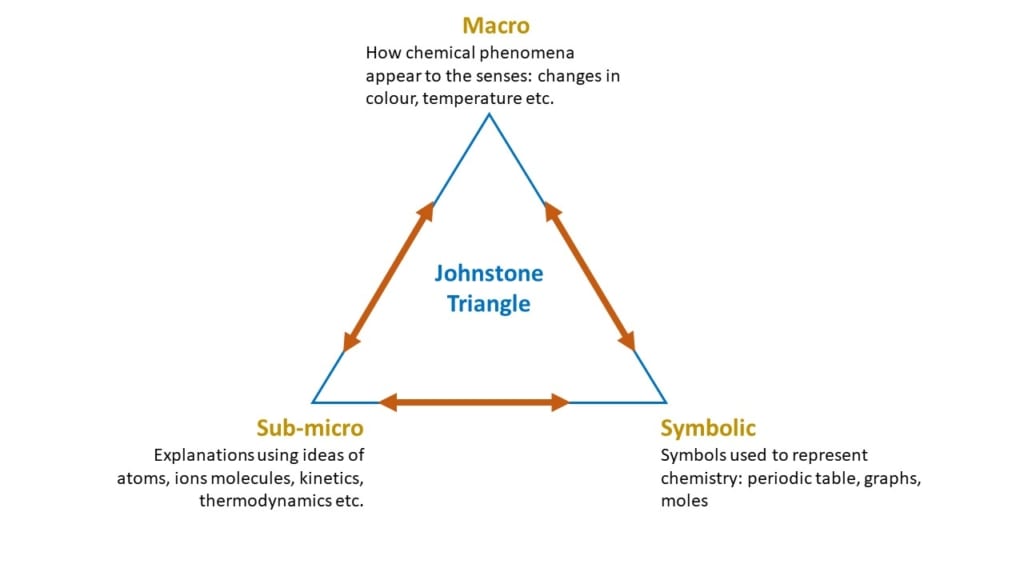
“To understand chemistry, students need to work at three levels. These are, the macroscopic level (observations), the sub-microscopic level (particles), and the symbolic level (equations). This is known as Johnstone’s Triangle.
As relative experts, teachers have internalised the differences and connections between these levels, and can easily switch between them. Students, as novices, have to contend with all three levels, often at once, without understanding the links between the ideas we are presenting. Simply put, they can find chemistry overwhelming.
Cognitive Load Theory
This theory builds upon the widely accepted model of human information processing shown in figure 2 (below) [3] (this was published by Richard Atkinson and Richard Shiffrin in 1968.)
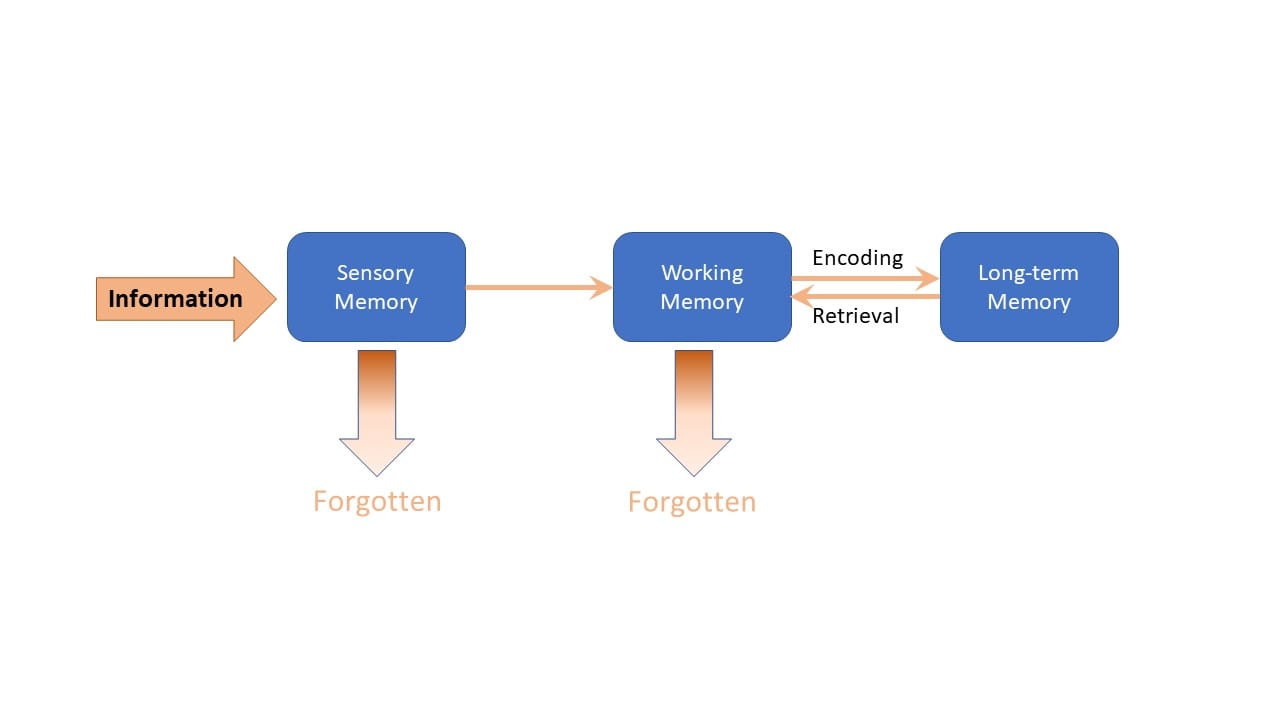
It describes the process as having three main parts: sensory memory, working memory and long-term memory. Since then, many researchers have added to our understanding of this concept, but the basic model remains the same.
Information is first received through our sensory memory, mainly through auditory and visual systems.
It is held there briefly until it enters our working memory.
Working memory can only handle a small number of items and for a short period of time (up to about 9 for about twenty seconds).
Given this fact, it is important that the information we are trying to share with students is clear and does not contain irrelevant, ‘extraneous’ information.
The third pillar of our cognitive architecture is long-term memory, which is where learning takes place.
The goal of teaching and learning is to pass information through these three memory types in order for students to be able to retain and transfer information in the future.
The amount of information contributes to this cognitive load. So when designing a course or lesson, you have to decide how much new information can be introduced at one time. The cognitive load is divided into three categories:
Intrinsic load – caused by the natural complexity of the information that must be processed. There is not a great deal that can be done about the task but intrinsic load is dependent on the learner’s level of expertise. If something is difficult, then it is difficult but structuring tasks by using simple terminology and clear examples can help to minimize intrinsic load.
Extraneous load – Caused by irrelevant or extraneous information that gets in the way of the important bits. Keeping the message focused on the learning, free from distractions, allows our working memory to perform more efficiently and encourages the flow of information on to our long-term memory.
Germane load – is the work put into transferring learning to the long-term memory. This is the effective cognitive load: the amount of intrinsic load minus extraneous load.
Effective instructional design tries to limit the intrinsic load to what is manageable and reduce the extraneous load as far as is possible, therefore increasing germane load. The learning we want takes place via this germane cognitive load.
Further information can be found here.[4]
An effective way of trying to address this problem in the context of practical work is to try to ensure that instructions are a clear as possible. David Paterson has been adapting some of the practical activities he uses with various classes to integrate the diagrams and the textual instructions into ‘integrated-instructions’. (He has made these available for all to use under a Creative Commons license)
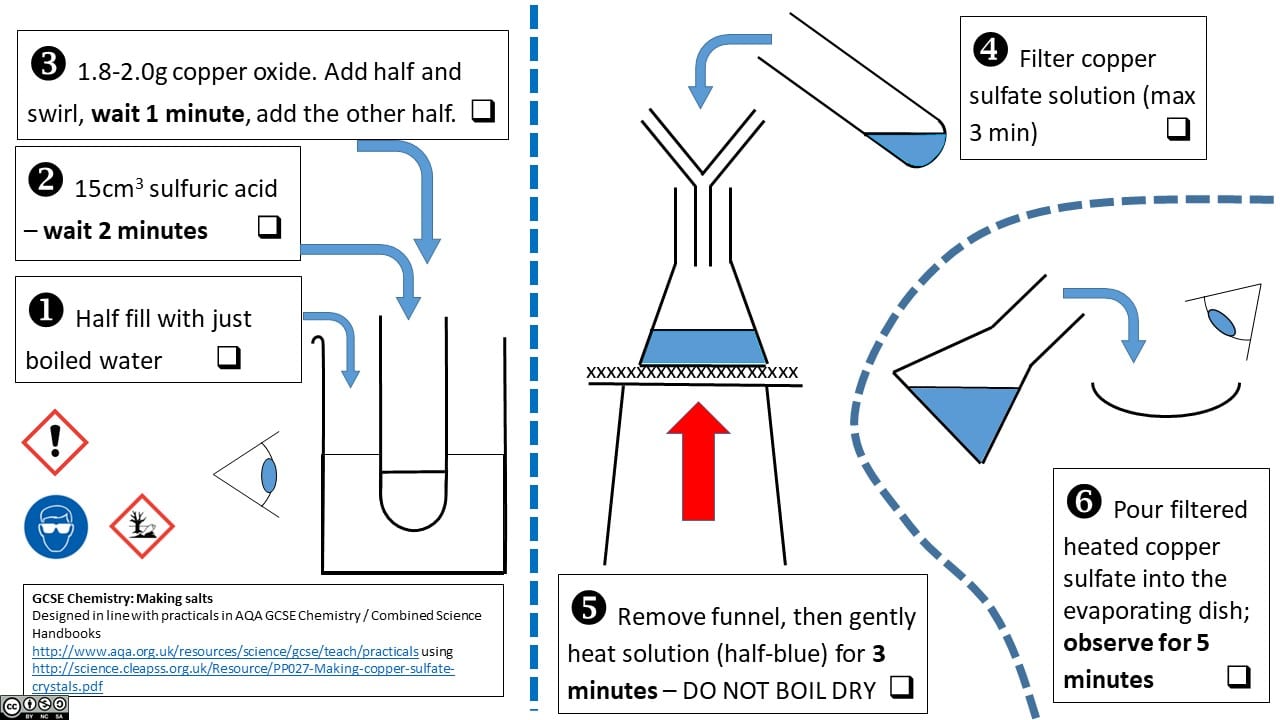
Consider the following sets of instructions for the same experiment: the production of copper sulphate by neutralising sulphuric acid with copper oxide.
1 – How the instructions might have appeared in a 19th century book.
(Wear eye protection) Half fill a beaker with just-boiled water. Add 15cm3 of sulphuric acid to a boiling tube and wait 2 minutes. Add 0.9-1.0g of copper oxide, swirl to mix and wait for 1 minute. Add another 0.9-1.0g of copper oxide. Filter the mixture into a conical flask. Gently heat the filtrate in the flask for 3 minutes. Do not boil dry. Pour the solution into an evaporating dish and observe for 5 minutes.
2 – How they might appear today
3 – Dave Paterson’s integrated instruction sheet for the same experiment
Conclusion
As with everything in education, there is no ‘one-size-fits-all’ method of producing instructions that will suit all situations. But we think this approach does bear considering in at least some situations.
The use of more diagrams and less text is a great advantage for pupils with poor written language skills: pupils with dyslexia and those with English as a second language are possible examples.
For those of you who either want to reduce printing and paper usage or simply are happier with a technology-based solution, it is possible to produce an animated version of these instruction sheets just using powerpoint.
We have some examples of this sort of instruction sheet on other tabs on ths page where you can also find an example of a powerpoint version for the experiment above. (You can also find many examples on Dave Paterson’s website)
This approach is applicable to other subjects as well – something we will visit in future articles.
[1] https://dave2004b.wordpress.com/
[2] https://eic.rsc.org/feature/making-practical-work-more-effective/3008027.article
[3] Atkinson, R.C. and Shiffrin, R.M. (1968). ‘Human memory: A Proposed System and its Control Processes’. In Spence, K.W. and Spence, J.T. The psychology of learning and motivation, (Volume 2). New York: Academic Press. pp. 89–195
[4] A Comparison of Three Measures of Cognitive Load: Evidence for Separable Measures of Intrinsic, Extraneous, and Germane Load. (Journal of Educational Psychology, 2008)
Apart from the one for electrode potentials, these are all versions (slightly amended) of ones Dave Paterson produced for GCSE assessed practicals. The first slide of each gives the Creative Commons License details and the second one has the instruction sheet itself.
As we move towards a more electronic based system of work, the need to stick to the limitations of paper become less. It is possible to create sinmple animations in powerpoint. I have used this to create an animated version of the instructions for making copper sulphate from above.
You can download the powerpoint to see how it is done from here (Animated CuSO4)
If you export it as a powerpoint show .ppsx (rather than the normal .pptx) then students only see the slideshow. Each step in the finished file is advanced by a click . (WordPress won’t let me upload a ppsx file, I’m afraid)
So you can see what it looks like, I am putting a video capture version below.
